STEMI DTU™ RCT

ST-segment Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Randomized Controlled Trial (RCT)
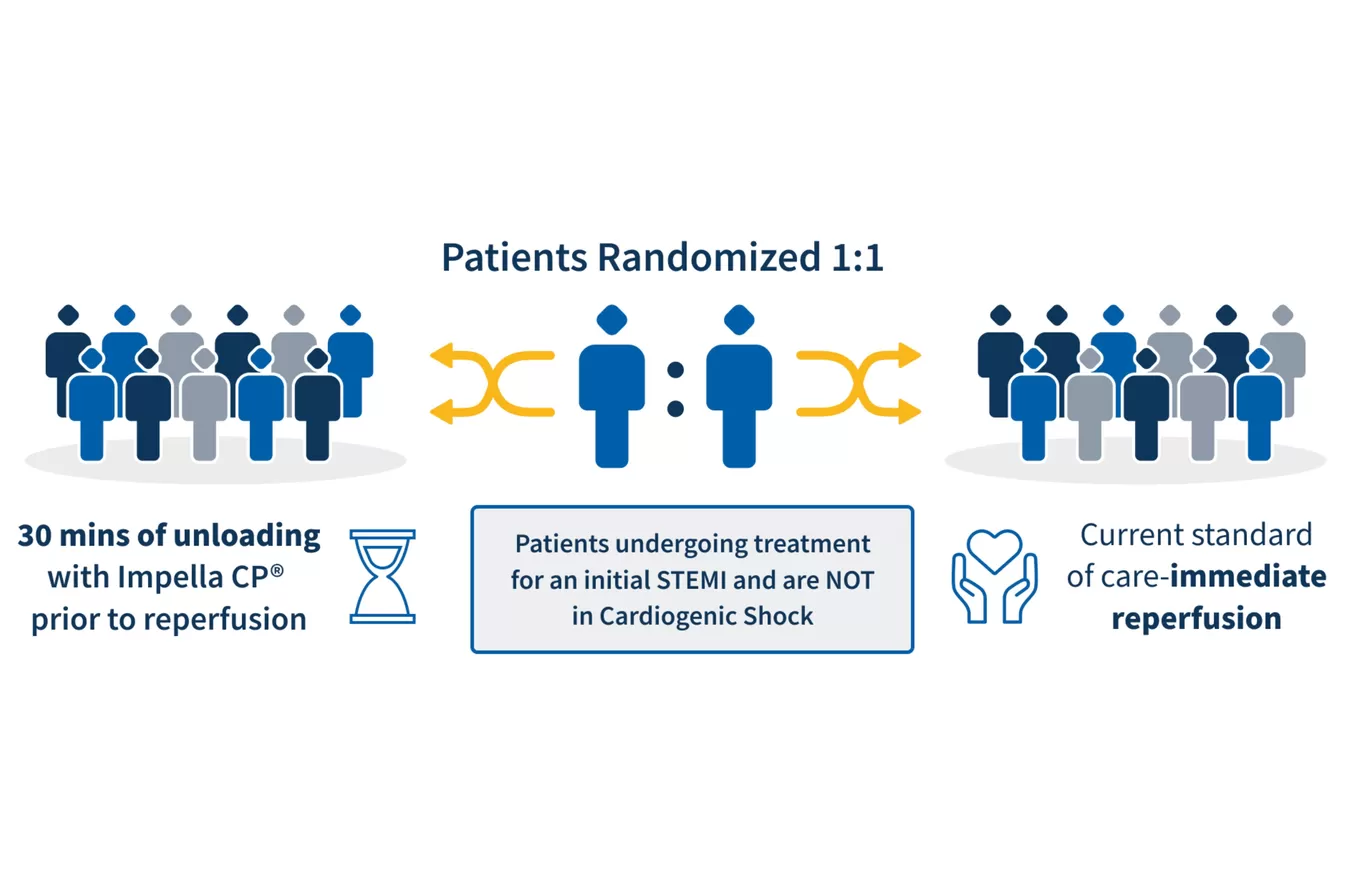
This pivotal trial builds on the success of the STEMI DTU safety and feasibility RCT, confirming the safety and feasibility of a 30-minute left ventricle unloading before reperfusion following a STEMI heart attack.

Trial Objective:
To demonstrate the safety and effectiveness of primary left ventricular unloading and evaluate whether using Impella CP® for 30 minutes prior to a catheterization procedure has the potential to reduce the damage to the heart caused by a heart attack, compared to the current standard of care.
Key Inclusion Criteria
- Age 18 to 85 years
- First myocardial infarction
- Acute anterior STEMI with ≥2 mm in 2 or more contiguous anterior leads or ≥4 mm total ST-segment deviation sum in the anterior leads V1-V4 AND anterior wall motion abnormality noted on a diagnostic quality left ventriculogram or echocardiogram
- Patient presents to hospital between 1-6 hours of ischemic pain onset
- Patient indicated for PPCI
Top 5 Exclusion Criteria
- Patient transferred from an outside hospital (OSH) where invasive coronary procedure was attempted (including diagnostic catheterization)
- Unwitnessed cardiac arrest OR ≥30 minutes of CPR prior to enrollment OR any cardiac arrest with impairment in mental status, cognition or any global or focal neurological deficit
- Administration of fibrinolytic therapy within 24hrs prior to enrollment
- Inferior STEMI or suspected right ventricular failure
- Cardiovascular History: Prior CABG or LAD PCI
Other important safety information can be found at https://www.abiomed.com/important-safety-information
Learn More about STEMI DTU
NPS-4320