Patient Identification
Bobbi Chapman: Tackling Healthcare Disparities
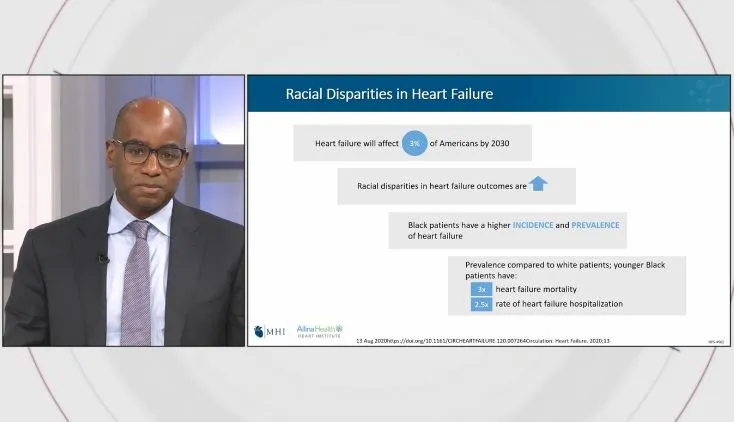
Bobbi Chapman, M.D., discusses how Abiomed is tackling healthcare disparities. “We don’t have good representation of Black Americans, Hispanics, or even women in our clinical trials,” Chapman explains, highlighting the need to better appreciate the true prevalence of disease in these underrepresented groups and target them for clinical trials to ensure that they have safe and effective drugs and devices.
Dr. Chapman briefly discusses the Diverse and Equitable Participation in Clinical Trials (DEPICT) Act that was passed with bipartisan support in 2022 to strengthen diversity in clincial trials. She emphasizes the importance of looking at age, race, sex, ethnicity, geography, and socioeconomics when setting enrollment targets for clinical trials.
Dr. Chapman highlights several strategies for diversifying clinical trial enrollment, beginning with clinical trial leadership. “We need trial leadership who reflect the patients we want to study, and that begins with your national principal investigators and your steering committee.” Other strategies include selecting sites that both serve and recruit underrepresented patients; investing in and recruiting underrepresented physicians for clinical trials; facilitating follow-up by addressing issues such as transportation challenges; improving community engagement; educating patients and providers about clinical trials; and improving barriers throughout the clinical trial lifecycle from protocol development through follow-up requirements.
Dr. Chapman addresses the importance of recognizing that regional differences impact enrollment targets, especially in US trials that include European sites. She explains the importance of intentionally developing diversity action plans for every clinical trial, creating clinical trial dashboards to provide transparency about overall trial enrollment, and sharing best practices among trial sponsors. She concludes by highlighting practices at Abiomed, including patient advisory groups, internal educational initiatives, and the development of study dashboards across all clincial trials to show enrollment metrics for age, race, ethnicity and sex, “so every member of the internal and external clinical team knows where we stand and where we need to go with enrollment of these underrepresented groups.”
NPS-5147