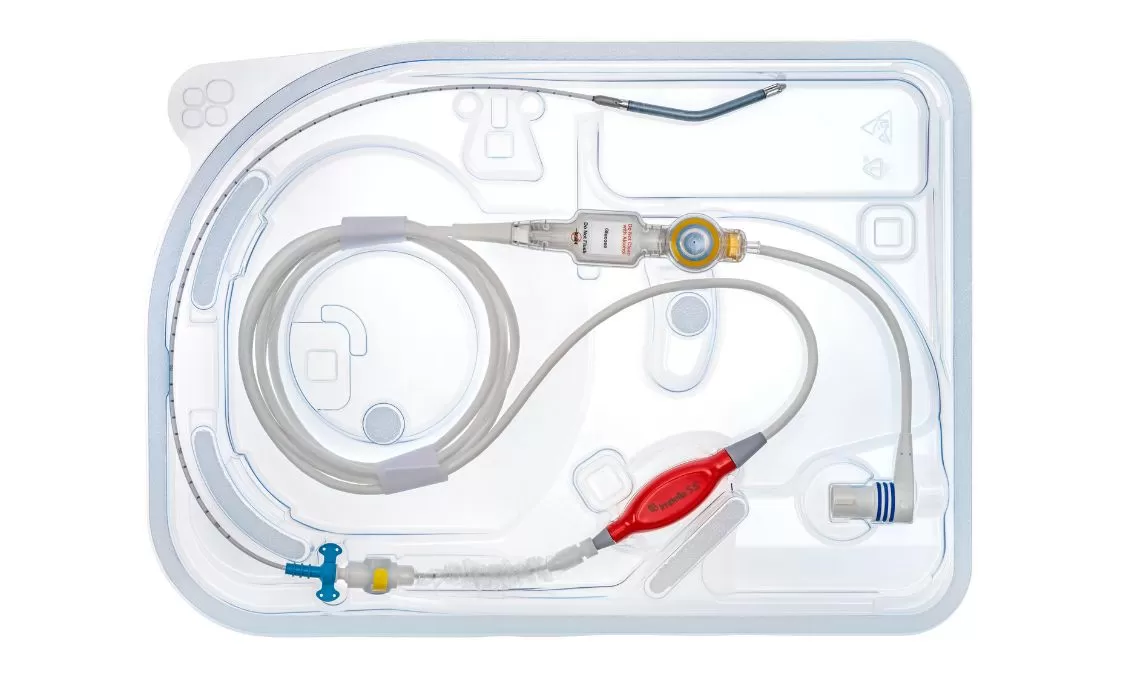
Impella 5.5®
Overview
Impella 5.5 with SmartAssist delivers full cardiac support with maximum unloading, allowing the heart to rest and recover.
It is a microaxial, surgically implanted heart pump that unloads the left ventricle, reduces ventricular work, and provides the circulatory support necessary to allow recovery and early assessment of residual myocardial function. It is designed for long-duration support and enables ambulation to optimize recovery while using real-time SmartAssist intelligence.
Clinical Evidence
A study published in the journal ASAIO examined the outcomes of the first 55 patients treated with Impella 5.5 with SmartAssist at Cleveland Clinic, Hackensack University Medical Center/Hackensack Meridian Health and Cedars-Sinai Medical Center. Study authors and cardiac surgeons Ed Soltesz, MD, Mark Anderson, MD, and Danny Ramzy, MD, conclude Impella 5.5 with SmartAssist is clinically appropriate for a number of challenging scenarios, including AMI cardiogenic shock.1
-
84%
Survival rate1
-
76%
Survivors recovered native heart function1
View an Animation Depicting the Primary Steps for Axillary Insertion of Impella 5.5 with SmartAssist
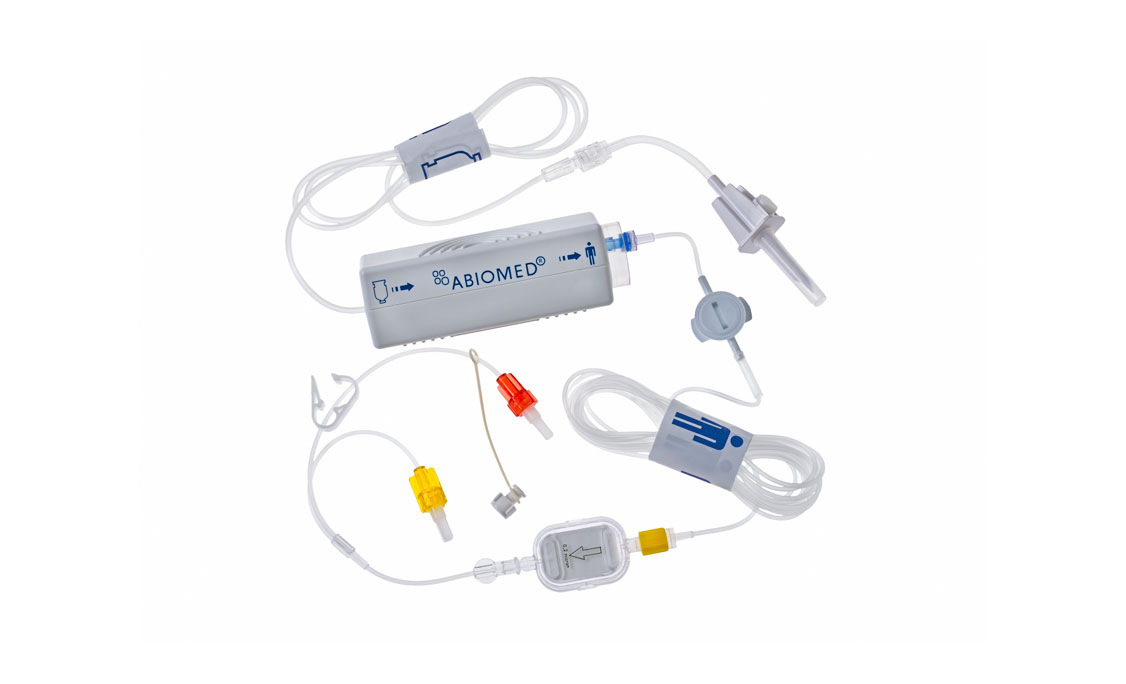
Accessories
Additional Resources
Indications for Use
The Impella 5.5® with SmartAssist® System is a temporary ventricular support device intended for short term (14 days) use and indicated for the treatment of ongoing cardiogenic shock that occurs immediately (< 48 hours) following acute myocardial infarction or open heart surgery or in the setting of cardiomyopathy, including peripartum cardiomyopathy, or myocarditis as a result of isolated left ventricular failure that is not responsive to optimal medical management and conventional treatment measures (including volume loading and use of pressors and inotropes, with or without IABP). The intent of Impella System Therapy is to reduce ventricular work and to provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function.
The Impella 5.5® with SmartAssist® Catheter is contraindicated for use with patients experiencing any of the following conditions: Mural thrombus in the left ventricle; Presence of a mechanical aortic valve or heart constrictive device; Aortic valve stenosis/calcification (equivalent to an orifice area of 0.6 cm2 or less); Moderate to severe aortic insufficiency (echocardiographic assessment graded as ≥ +2); Severe arterial disease precluding placement of the Impella System; Presence of an Atrial or Ventricular Septal Defect (including post-infarct VSD); Significant right heart failure*; Left ventricular rupture*; Cardiac tamponade*; Combined cardiorespiratory failure*. Those with an asterisks (*) apply to the cardiogenic shock indication.
Acute renal dysfunction, Aortic valve injury, Bleeding, Cardiogenic shock, Cerebral vascular accident/Stroke, Death, Hemolysis, Limb ischemia, Myocardial infarction, Renal failure, Thrombocytopenia and Cardiac or Vascular injury (including ventricular perforation).
In addition to the risks above, there are other WARNINGS and PRECAUTIONS associated with Impella devices.
Visit www.abiomed.com/impella and to learn more.
Footnotes
- Ramzy, D., et al. (2020). New Surgical Circulatory Support System Outcomes. ASAIO Journal.
IMP-1703