Impella RP®
Overview
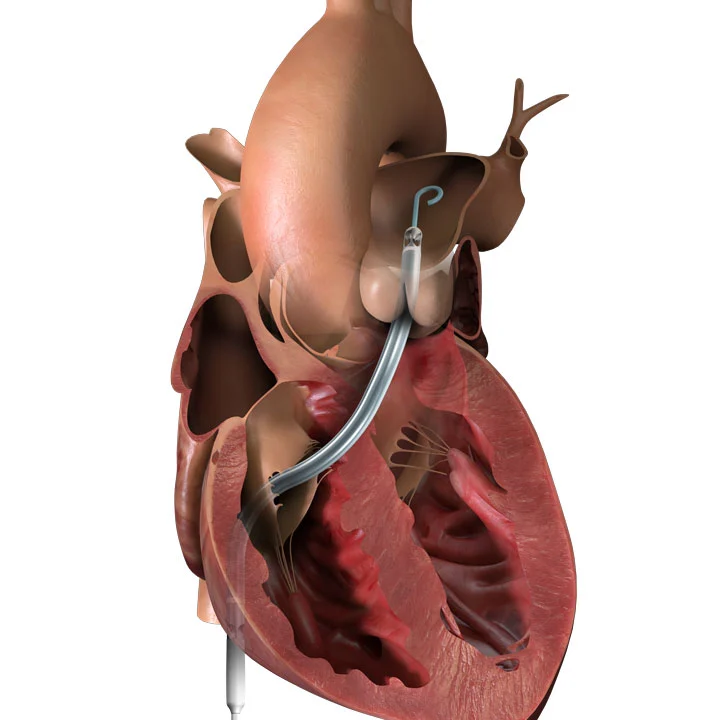
The Impella RP heart pump is a percutaneous device for right heart support.
This technology pumps blood from the inferior vena cava to the pulmonary artery. The pump is inserted with venous access and advanced over a wire into the pulmonary artery using standard catheterization techniques.
- Provides circulatory assistance for up to 14 days
- Delivers flow > 4.0 L/min of blood
- Enables biventricular support when the left side is already supported with a left-sided Impella® device
Indication and Safety Information EU
For indication and safety information please refer to the instruction for use.
IMP-3058