Clinical Evidence
Clinical Safety and Efficacy of Protected PCI
- Extensive data, including randomized controlled trial data and FDA-reviewed studies, support Impella® systems safety and efficacy.1,2,7
- Impella 2.5® and Impella CP® heart pumps maintain patient hemodynamics which may allow for more complete revascularization and reduction in major adverse coronary and cerebrovascular events (MACCE).8
- Impella heart pumps are associated with reduction of kidney injury during high-risk PCI.3
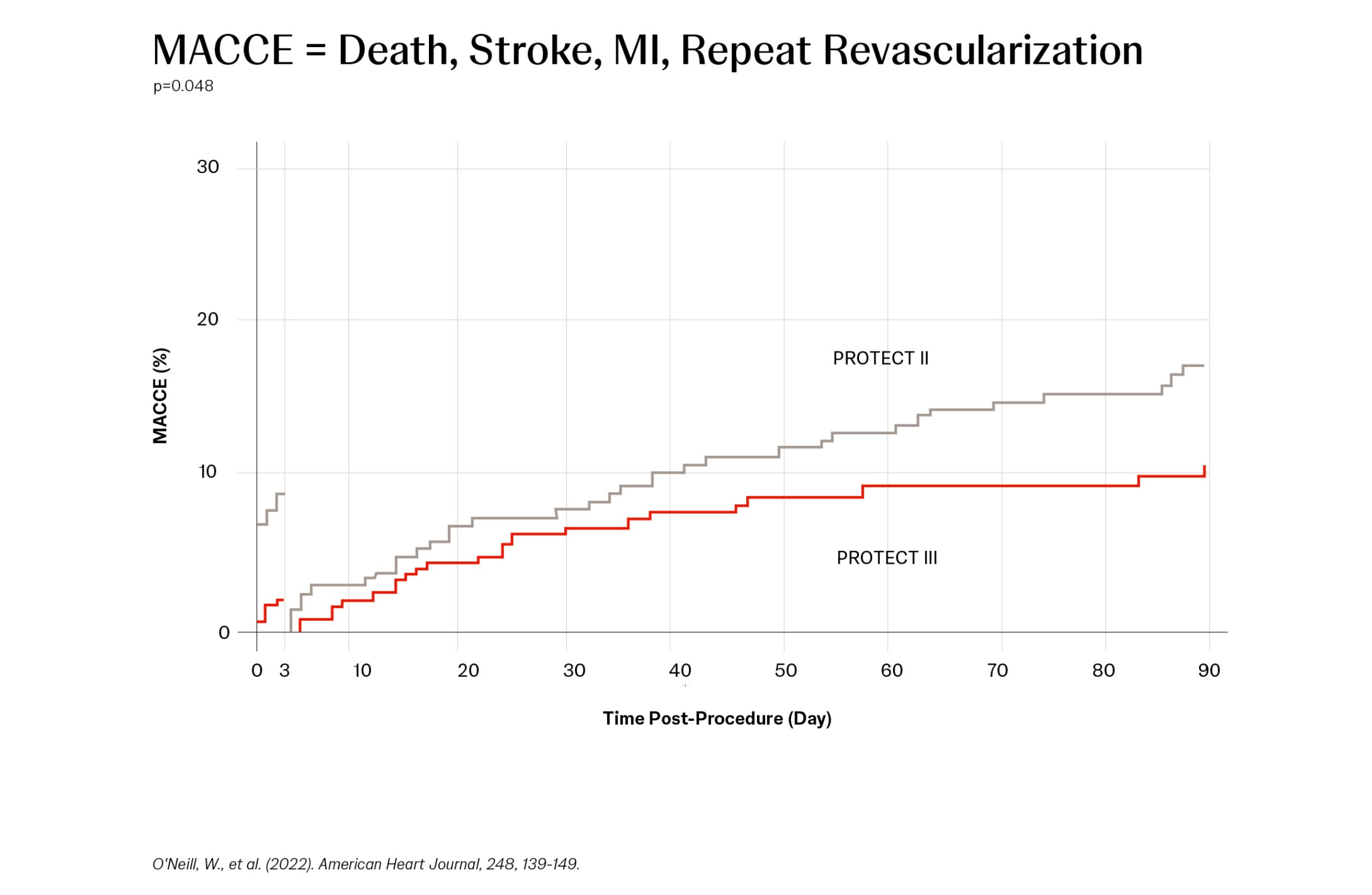
- PROTECT III, the most contemporary data, continues to show a reduction in MACCE with Impella2 pumps
Clinical Evidence Insights About Impella Heart Pumps in the Field of Protected PCI
All MACCE to 90 Days
Complete Revascularization Associated with Improved Outcomes
Benefits in high-risk acute coronary syndrome (ACS) patients at 1 year4
- 71% risk reduction in all-cause death
- 27% risk reduction in major adverse cardiovascular events (MACE)
- 41% risk reduction in myocardial infarction (MI)
Benefits in high-risk STEMI patients at 3 years5
- 26% risk reduction in cardiovascular (CV) death/MI
- 47% risk reduction in CV death/MI/revascularization
Meta-analysis of benefits in 11 high-risk STEMI studies6
- 29% risk reduction in CV death/MI


EF Improvement Following Contemporary High-Risk PCI - Restore EF
RESTORE EF is a prospective multicenter study that assessed 90-day LVEF improvement as the primary endpoint and changes in NYHA HF and CCS angina symptom classification as the secondary endpoint. It found significant LVEF improvement at 90 days and significant reduction in NYHA HF and CCS angina Class III/IV symptoms.


Revascularization Completeness with pVAD vs IABP
In a third-party analysis conducted by the York Health Economics Consortium individual patient data (IPD) from contemporary studies—PROTECT II randomized controlled trial, PROTECT III, and RESTORE EF—was evaluated to test the hypothesis that percutaneous coronary intervention (PCI) with Impella® heart pump support may be associated with a greater extent of revascularization and, thus, may contribute to superior clinical outcomes compared to PCI of equivalent complexity utilizing intra-aortic balloon pump (IABP).
Protected PCI Improves Quality of Life
Clinical data demonstrate that Protected PCI improves quality of life by increasing ejection fraction, reducing NYHA class, reducing adverse events, and reducing acute kidney injury requiring dialysis.3,7,8
“PROTECT III clearly demonstrates how the evolution and adoption of Impella best practices can lead to an improvement in safety and MACCE.”
Clinical Trials
References
- Dixon, S.R., et al. (2009). JACC Cardiovasc Interv, 2(2), 91-96.
- Moses, J., et al. (2020). Presented at TCT Connect 2020.
- Flaherty, M.P., et al. (2017). Circ Res, 120(4), 692-700.
- Généreux, P., et al. (2012). J Am Coll Cardiol, 59(24).
- Mehta, S.R., et al. (2019). N Engl J Med, 381(15):, 1411-1421.
- Bainey, K.R., et al., (2020) JAMA Cardiol.
- O’Neill, W.W., et al. (2012). Circulation, 126(14), 1717-1727.
- Dangas, G.D., et al. (2014). Am J Cardiol, 113(2), 222-228.
Discover More About Protected PCI
NPS-2624