Impella CP®
Overview
The Impella CP® with SmartAssist Heart Pump® marks a significant advancement in circulatory support technology, offering an innovative solution for patients with severe cardiac conditions.
This next-generation intravascular microaxial blood pump uses real-time intelligence to optimize patient outcomes and enhances the positioning, management, and weaning of the device. Optimally positioned via a percutaneous insertion through the femoral artery, the Impella CP with SmartAssist delivers critical support directly to the left ventricle, reducing cardiac workload and improving systemic perfusion with a peak flow of up to 4.3 L/min. Designed for high-risk percutaneous coronary interventions (PCI) and post-acute myocardial infarction cardiogenic shock (AMI CS), the device supports the circulatory system for up to five days.
Product Specifications
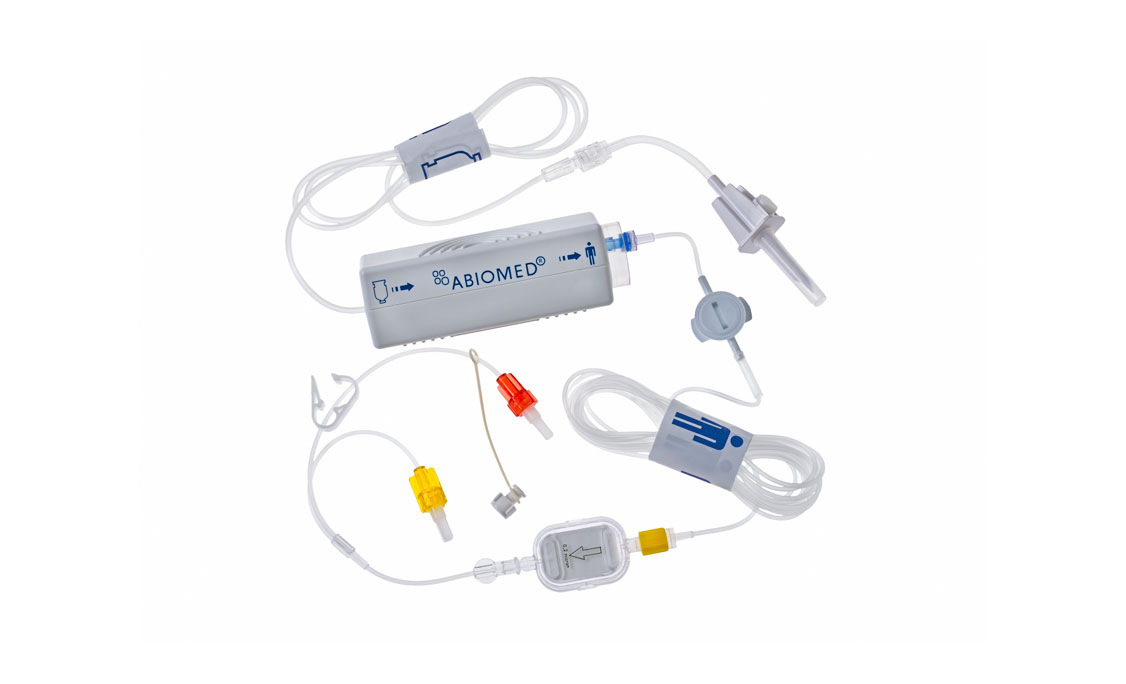
- Cannula: Polyurethane coated nitinol with a 145-degree angle
- Easy Guide Lumen: Red loading lumen to ease guidewire loading
- Catheter Shaft: Polyurethane catheter with nitinol backbone and triple internal lumens for pressure, purge and electrical signal
- Position Sensor: Optical pressure sensor located immediately distal to the outlet, provides a pressure reading indicating aortic pressure only when both the outlet and sensor are located within the aorta
- Repositioning Unit: Graduated shaft from 9 Fr to 13 Fr with StatLock®* compatible suture pads and anti-contamination sleeve. Guidewire reaccess sheath maintains guidewire access to arteriotomy. 13 Fr section is 10cm in length
- Red Impella Plug: Connections - 1 luer connection for purge fluid, 1 electrical connection direct to Automated Impella® Controller
Features to Improve Patient Outcomes
Comprehensive Overview of Clinical Evidence and the SmartAssist Platform
Additional Resources
Accessories and Ordering Information
Discover Our Impella Portfolio
Indication and Safety Information EU
The Impella (intracardiac pump for supporting the left ventricle) is intended for clinical use in cardiology and in cardiac surgery for up to 5 days for the following indications, as well as others:
· The Impella is a circulatory support system for patients with reduced left ventricular function, e.g., post-cardiotomy, low output syndrome, cardiogenic shock after acute myocardial infarction, or for myocardial protection after acute myocardial infarction
· The Impella may also be used as a cardiovascular support system during coronary bypass surgery on the beating heart, particularly in patients with limited preoperative ejection fraction with a high risk of postoperative low output syndrome.
· Support during high risk percutaneous coronary intervention (PCI)
· Post PCI
· Mechanical aortic valves, severe aortic valvular stenosis or valvular regurgitation
· Hematological disorder causing fragility of the blood cells or hemolysis
· Hypertrophic obstructive cardiomyopathy (HOCM)
· Aneurysm or necrotomy or severe anomaly of the ascending aorta and / or the aortic arch
· Mural thrombus in the left ventricle
· Ventricular septal defect (VSD) after myocardial infarction
· Anatomic conditions precluding insertion of the pump
· Other illnesses or therapy requirements precluding use of the pump
There are risks of complications with every procedure using a blood pump. These include among others:
· Acute renal dysfunction
· Aortic valve injury
· Cardiogenic shock
· Cerebral Vascular Accident/Stroke
· Myocardial infarction
· Renal failure
· Thrombocytopenia
· Hemolysis
· Bleeding
· Limb Ischemia
· Immune reaction
· Embolism, thrombosis
· Cardiac or vascular injury (including vertricular perforation)
· Positioning problems causing hemolysis or reduced haemodynamic support
· Infection and septicemia
· Dislocation of the pump
· Cardiovalvular injuries due to extreme movement of the suction cannula in relation to the cardiac valve or as a result of attachment by suction of the pump to the valve system following incorrect positioning
· Endocardiac injuries as a result of attachment of the pump due to suction
· Pump failure, loss of pump components following a defect
· Patient dependency on the pump after use for support
In addition to the risks above, there are other WARNINGS and PRECAUTIONS associated with Impella devices. For more information please see the Instructions for Use Manuals.
NOTE
*StatLock is a trademark of Becton, Dickinson and Company
**Only for use in ventricularized pumps
1. Fincke 2004 J Am Coll Cardiol_CGS SHOCK registry (v2.0)
IMP-3059